Watch to learn how offering Axonics SNM can help you treat more patients in your practice.
Sacral Neuromodulation (SNM) Therapy is a long-lasting treatment option indicated to help patients who are suffering with Overactive Bladder, Fecal Incontinence and Urinary Retention, allowing you to help more patients in your practice.
80% of Overactive Bladder (OAB) patients report discontinuation of their incontinence medications,4 and yet only a small fraction of these patients proceed to third-line therapies.5
Although Botox® is the most prescribed third-line therapy for OAB5, studies show that over 60% of patients who are administered Botox discontinue after 2 - 3 injections.6
![Over 60% Discontinue Botox Treatment after 2 - 3 injections [6]](/images/over-60-botox.png)
![Over 60% Discontinue Botox Treatment after 2 - 3 injections [6]](/images/m-over-60-botox.png)
Axonics Sacral Neuromodulation Therapy is also an effective treatment option for patients who have tried Botox® therapy but found it unsuccessful or discontinued their treatment.7
Axonics has improved the patient and provider experience with SNM therapy, allowing patients to receive long-lasting relief without repeat office visits.
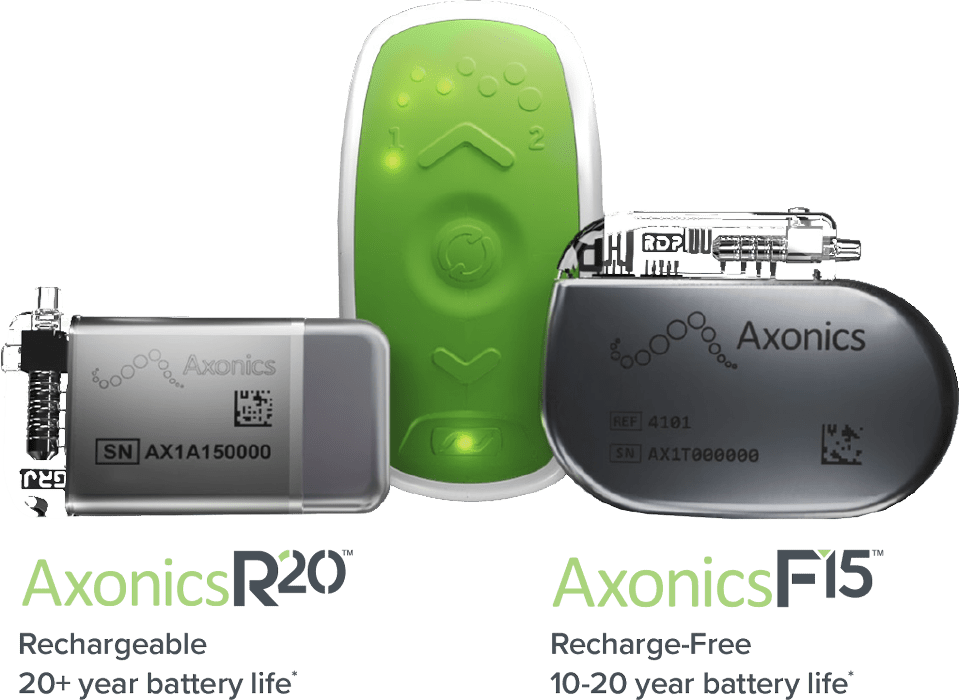
In the 20 year lifespan of an Axonics SNM system*, a Botox patient would have to receive 40 injections.8
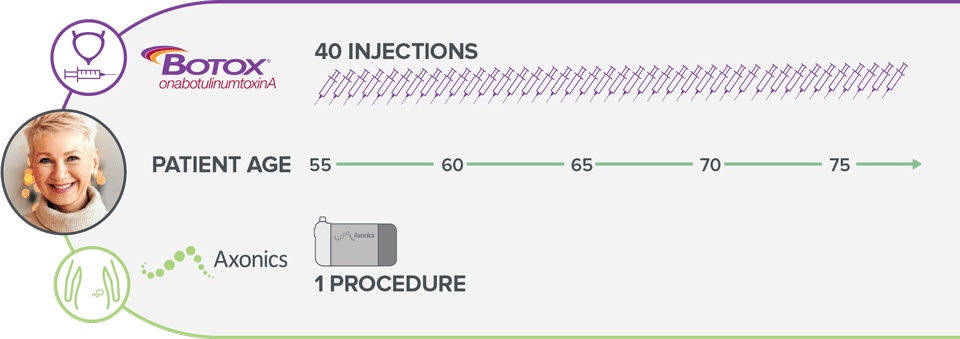
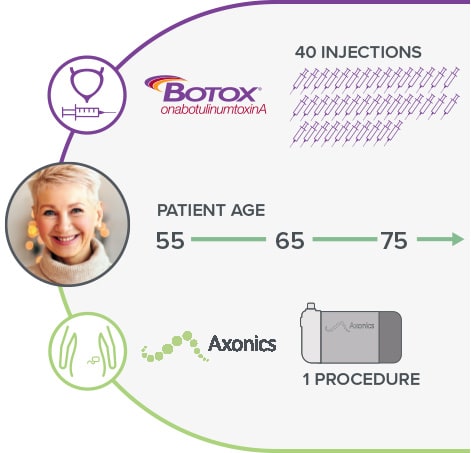
*Depending on therapy settings
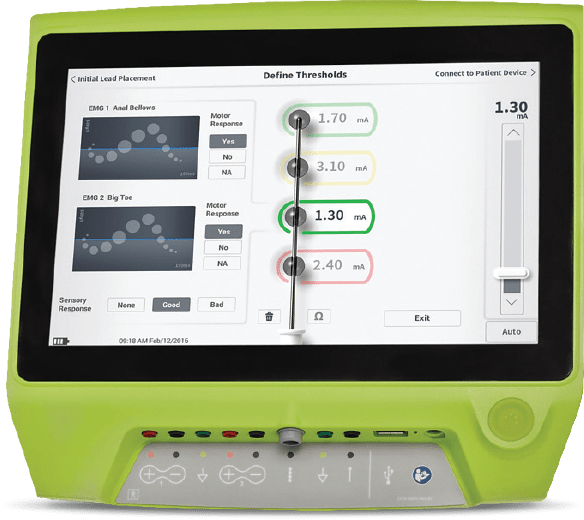
The Smart programming algorithm personalizes a patient’s stimulation parameters to their lead placement and takes the “guesswork” out of programming.
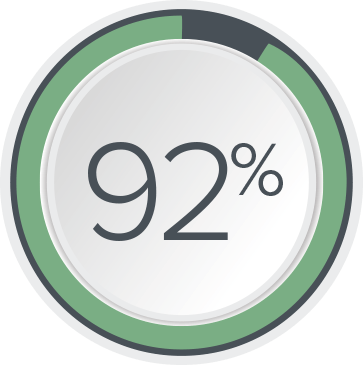
In a clinical study, 92% of patients stayed on their initial program at 6 months and experienced clinically meaningful improvement* in their symptoms.9
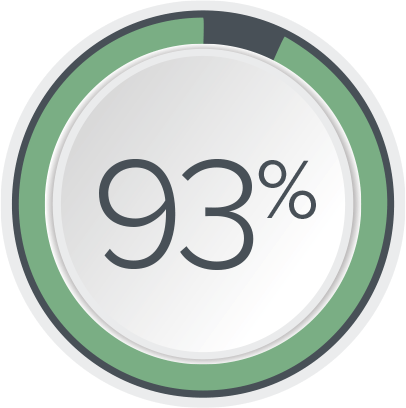
of patients had a ≥50% reduction in UUI symptoms at 2 years10

of patients were satisfied with their therapy10
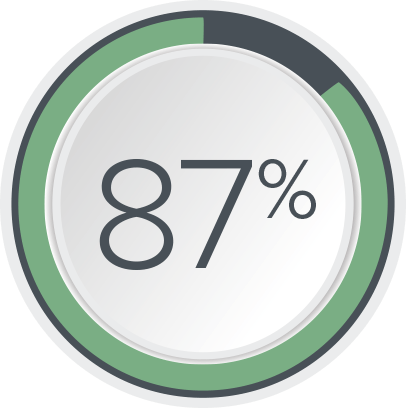
of FI patients had ≥50% reduction in FI episodes10
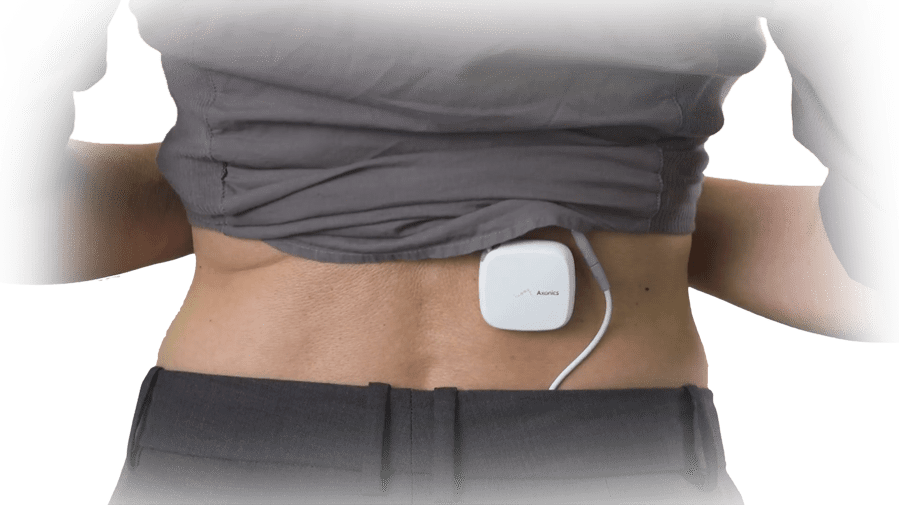
Axonics SNM offers the ability to trial the therapy and assess symptom improvement prior to having your patient undergo the full implant procedure.
Indications: Axonics SNM Therapy for urinary control is indicated for the treatment of urinary retention and the symptoms of overactive bladder, including urinary urge incontinence and significant symptoms of urgency-frequency alone or in combination, in patients who have failed or could not tolerate more conservative treatments. Axonics SNM Therapy for bowel control is indicated for the treatment of chronic fecal incontinence in patients who have failed or are not candidates for more conservative treatments.
Contraindications: Axonics SNM Therapy is contraindicated for patients who have not demonstrated an appropriate response to test stimulation or patients who are unable to operate the Axonics SNM Systems.
Warnings: Implantation and use of the Axonics Systems incur risks beyond those normally associated with surgery, some of which may necessitate surgical intervention. These risks include, but are not limited to adverse change in voiding function (bowel and/or bladder), infection, pain or irritation at the implant site, lead or device migration, electrical shock, change in sensation or magnitude of stimulation which has been described as uncomfortable (jolting or shocking) by some patients, and heating or burns at the device site.
For more safety information about indications and potential risks, go to www.axonics.com/isi.
Precautions: The safety and effectiveness of Axonics Therapy has not been established for use in women who are pregnant or in delivery; for pediatric patients (under the age of 18 years for fecal incontinence and under the age of 16 years for overactive bladder and urinary retention); for patients with neurological diseases, such as multiple sclerosis or diabetes, or for bilateral stimulation.
Caution: U.S. federal law restricts this device to sale and use by, or on the order of, a physician.
Reference:
Botox is a registered trademark of Allergan, Inc.